
Introduction
Daratumumab, when added to standard of care regimens in relapsed and untreated myeloma, has consistently demonstrated significant improvements in response rates, induction of MRD negative responses and progression-free survival (PFS) while proving highly tolerable with minor increases in overall regimen toxicity. In non-transplant eligible patients daratumumab has been added in randomized studies to lenalidomide and dexamethasone (Rd) and bortezomib, melphalan and prednisolone (VMP) backbones, but not to the VCD regimen. Furthermore, the randomized studies excluded a significant proportion of patients with comorbidities so the benefit of daratumumab in a frail, elderly myeloma population remains untested.
Methods
Inclusion criteria included untreated patients with symptomatic myeloma who were considered ineligible for high-dose chemotherapy with autologous stem cell transplantation due to either age >65years or the presence of comorbidities. Any degree of renal impairment, including dialysis dependence, was allowed as were patients with a prior history of systemic malignancy that had been disease-free for 2 years. Patients were randomized 1:1 to receive VCD or VCDD. VCD consisted of nine 5-week cycles of V 1.3 mg/m2 SC on Days 1, 8, 15 and 22; C 300mg/m2 PO on Days 1, 8, 15 and 22 and D 20 mg PO on Days 1, 8, 15 and 22. VCDD consisted of nine 5-week cycles of VCD plus daratumumab 16 mg/kg IV on Days 1, 8, 15 and 22 of cycles 1 and 2, Days 1 and 15 of cycles 3 to 6 and Day 1 of cycles 7 to 9, followed by daratumumab maintenance 16 mg/kg IV every 4 weeks until progression. The primary endpoint was PFS with secondary endpoints being response rates, MRD, overall survival, toxicity and quality of life.
Results
A total of 129 patients were randomized, but 7 did not commence intended therapy. The following modified ITT analysis is based on the 122 randomized patients, 58 in the VCD group and 64 in the VCDD group, who received therapy. Baseline characteristics were balanced between the two arms. Median age was 76 years (range, 62-91yrs), with 19% being ≥80 years of age. 30% were female. ECOG performance status was 0 (34%),1 (26%), 2 (16%) and unknown (25%). ISS stage was I (16%), II (36%), III (23%) and unknown (24%). The estimated median potential follow-up is 12.6 months.
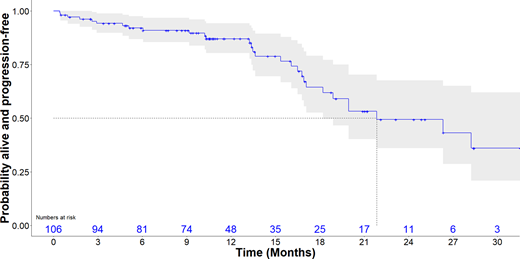
At the time of this report, the COVID-19 pandemic had impacted collection of site data. As a result, the following outcome data is provisional with a full data set to be available for presentation of the formal pre-planned interim analysis by the time of the ASH meeting. Overall response rate was 86% for VCD and 93% for VCDD. There was no significant difference between response rates after 4 cycles of induction for the VCD and VCDD arms: CR 3% vs 2%, VGPR 31% vs 41%, PR 51% vs 50%, MR 11% vs 7%, PD 3% vs 0%. Median PFS for the entire cohort (Fig A) was 21.8 months (95%CI 17.1-31.6 months). Median PFS for those treated with VCD was 18.9 months (95%CI 15.3-NR) and was 26.3 months (95%CI 17.1-31.6 months) for those treated with VCDD. In both arms combined, median PFS was 26.3 vs 21.9 months for those aged <75 vs ≥75 yrs, and not reached, 21.8 months and 19.9 months for those with ISS stage I, II and III, respectively.
19% of patients in the VCD group and 16% of patients in the VCDD group ceased therapy early, predominantly due to adverse events or death. SAEs during the induction period occurred in 44% and 52% of patients in the VCD and VCDD arms, respectively. There were 13 patients with SAEs due to infection in the VCD group and 20 in the VCDD group. Grade 3 or 4 peripheral neuropathy was uncommon, with only one case in the VCD arm.
Conclusions
The VCD schedule as detailed in this study appears efficacious, well tolerated and deliverable to an elderly myeloma population. The addition of daratumumab does not compromise chemotherapy delivery and may improve PFS. Formal interim analysis of the trial data will be presented at the meeting.
Mollee:Amgen: Membership on an entity's Board of Directors or advisory committees; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding. Reynolds:Novartis AG: Current equity holder in publicly-traded company. Janowski:Janssen: Membership on an entity's Board of Directors or advisory committees; BMS/ Celgene: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; AstraZenica: Consultancy. Quach:Amgen, Celgene, karyopharm, GSK, Janssen Cilag, Sanofi.: Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Honoraria; GlaxoSmithKline, Karyopharm, Amgen, Celgene, Janssen Cilag: Consultancy; Amgen, sanofi, celgene, Karyopharm, GSK: Research Funding. Campbell:Amgen, Novartis, Roche, Janssen, Celgene (BMS): Research Funding; AstraZeneca, Janssen, Roche, Amgen, CSL Behring, Novartis: Consultancy. Gibbs:Janssen, BMS/Celgene, Amgen, Takeda, Pfizer, Caelum, Abbvie and Eidos: Membership on an entity's Board of Directors or advisory committees. D'Rozario:Abbvie: Membership on an entity's Board of Directors or advisory committees; BMS/ Celgene: Membership on an entity's Board of Directors or advisory committees. Wallington-Beddoe:Amgen: Membership on an entity's Board of Directors or advisory committees. Weber:Amgen: Membership on an entity's Board of Directors or advisory committees. Spencer:Celgene, Janssen and Takeda: Speakers Bureau; AbbVie, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Consultancy; Amgen, Celgene, Haemalogix, Janssen, Servier and Takeda: Research Funding; AbbVie, Amgen, Celgene, Haemalogix, Janssen, Sanofi, SecuraBio, Specialised Therapeutics Australia, Servier and Takeda: Honoraria.
Daratumumab as initial treatment of myeloma
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal